MEDICAL DEVICE:CLINICAL TRIAL IN CHINA,EUROPE AND USA
REGULATORY SERVICE FOR MEDICAL DEVICE GLOBALLY
Company Introduction
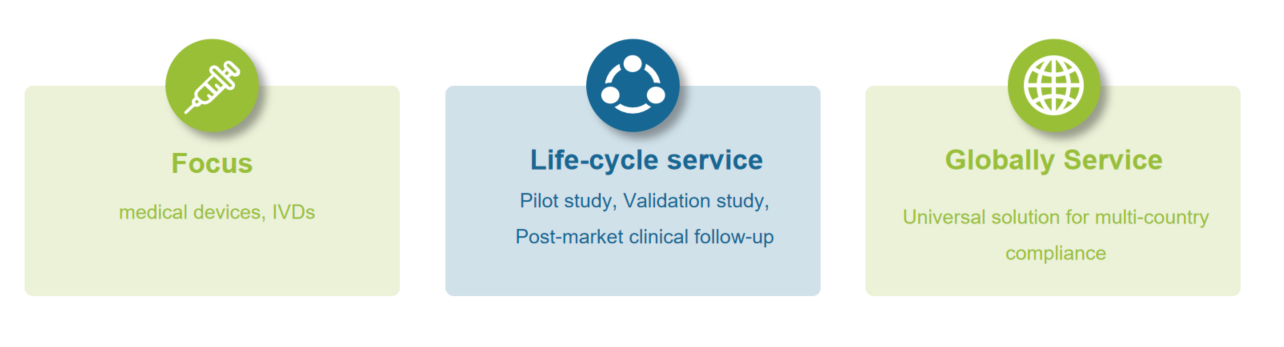
Care-real is a Medical Device Contract Research Organization (CRO).
Care-real provides Medical Device Registrants with: Global Clinical Trials & Clinical Evaluation, Regulatory Compliance, Software & Digitalization Services.
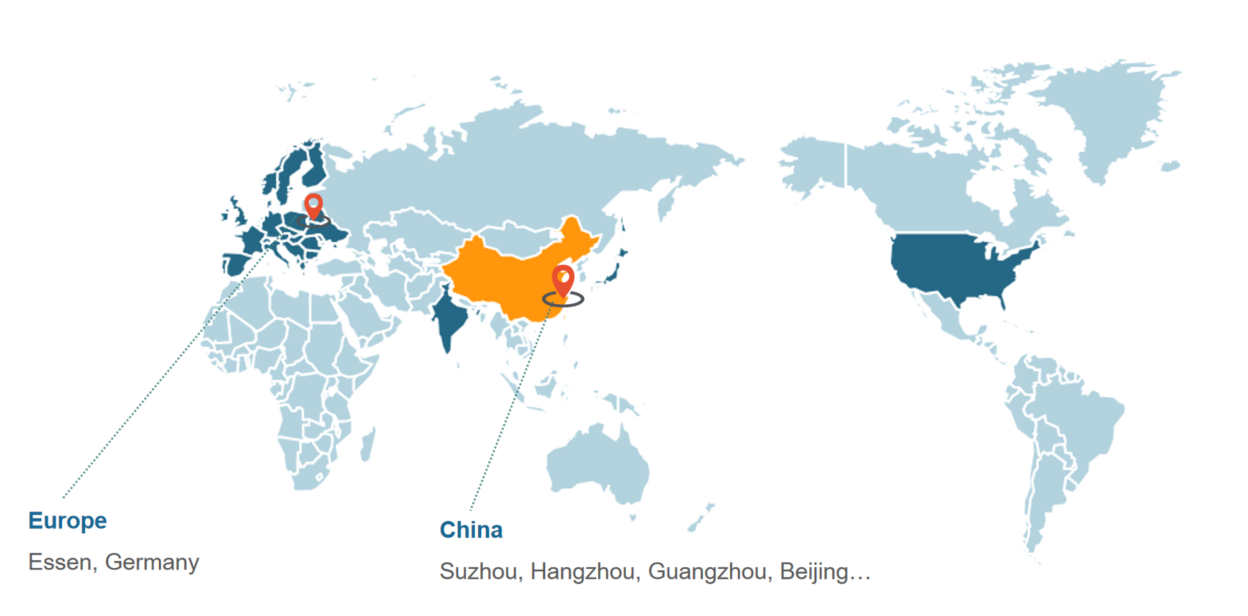
Care-Real Group
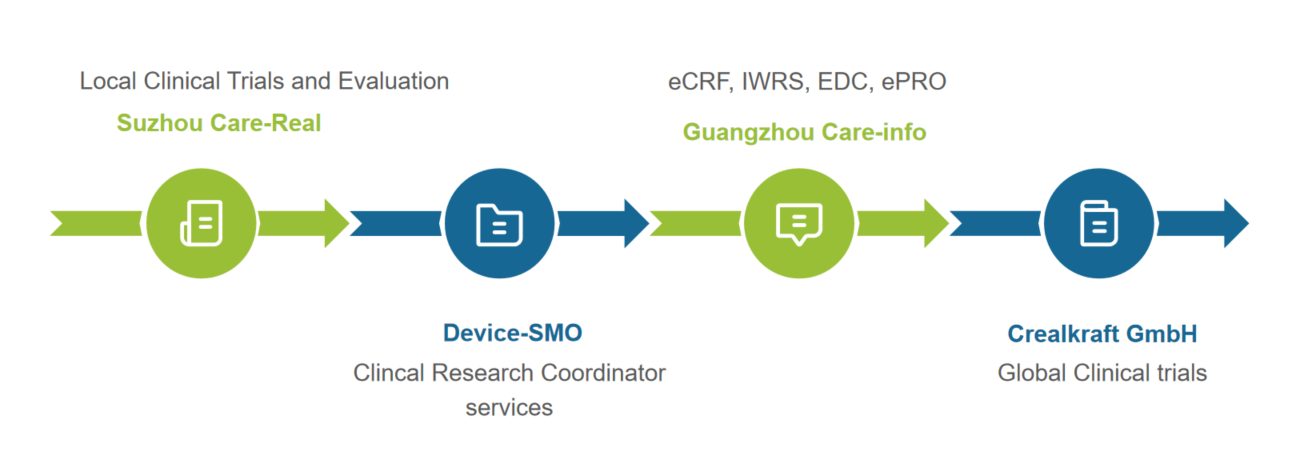
Service Scope

Clinical Evaluation (Study) Throughout the Entire Life Cycle of Medical Devices
Design and Development Planning & Input Concept Verification Study
Clinical Pain Point Analysis
Extensive Clinical Investigation
Medical Literature Retrieval and Novelty Search
Design and Development Implementation & Verification Exploratory Study
Phased Clinical Review
Clinical Usability Evaluation
Animal Experiment Preliminary Study
Design and Development Change / Post-Market Study
Product Iteration Clinical Investigation
Post-Market Clinical Study
Real-World Study
Design and Development Transfer & Confirmation Confirmatory Study
Summary of Clinical Usability
First-in-Man Clinical Trial (FIM)
Regulatory Clinical Trial
Clinical Evaluation of Same Kind Products
Domestic and Overseas Clinical Trials
Asia Clinical Research
Mainland China
Hong Kong, Macao and Taiwan Regions
Southeast Asia
Europe Clinical Research
Germany, France and 4 Nordic Countries
Italy, Greece, Portugal and Spain
Romania, Poland, Hungary
Americas Clinical Research
U.S. Clinical Research
Brazil Clinical Research
Chile Clinical Research
Domestic and Overseas Clinical Trials
Clinical Research Service Content
Medical Writing
Compliance with Multinational Regulations
Medical Writing
Case Report Form (CRF) Design
Medical Translation
Medical Report Writing
Monitoring Service
Site Selection
Rapid Initiation / Site Startup & Activation (SSU)
Ethics Submission
Monitoring and Quality Control
Project Management Service
Biostatistics Service
Statistical Analysis Plan (SAP) Writing
Data Cleaning
Data Statistical Analysis
Data Submission Format
Audit Service
Second-Party Audit Service
Independent Third-Party Audit
Mock Inspection
Trial System Guidance
Software Service
Clinical Trial Management System (CTMS)
Electronic Data Capture System (EDC)
Interactive Web Response System (IWRS)
Electronic Trial Master File (eTMF)
Full-Process Planning for Multinational Compliance Access
Compliance Pathway Planning
NMPA Registration Planning
Multinational Synchronized Access Pathway Planning
Clinical Evaluation Pathway Planning
Clinical Evidence Collection Planning
Testing and Verification Planning
Supplier Screening for Biological and Animal Testing
Electrical Safety and EMC Planning
Ergonomics and Cybersecurity Planning
Product Sterilization Planning and Supplier Screening
Compliance Consulting Service
Regulatory Consulting Service
Quality Management System Consulting
Clinical Evaluation Regulatory Consulting
Full-Process Compliance Consulting for Market Access
Training Service
Customized Training
Product Compliance Access Training
Quality Management System Training
Clinical Evaluation (Study) Training
Digital System for Clinical Research
01-EDC
Electronic Data Capture
Rapid Data Entry
Real-Time Data Cleaning
Audit Trail
02-eTMF
Trial Master Files
Real-Time Archiving
System Quality Control
Ensure Compliance & Completeness
03-IWRS
Interactive Web Response System
Multiple Randomization Methods
EDC & CTMS Integration
04-CTMS
Clinical Trial Management System
Risk and Cost-Based Management
Multi-System Interconnection & Closed-Loop
Full-Process Management System for Medical Device Manufacturing
01 MAH Collaboration Software
Production Management
Document Management
Market Release Management
Traceability Management
02 Manufacturer ERP System
Purchase, Sales and Inventory Management
Production Management
Report Management
Quality Management
03 Manufacturing Execution System (MES)
Production Management
Quality Management
Report Management
Workshop Management
04 Full-Process Information Management System
UDI Management
Adverse Event / Recall Management
Design and Development Management
Training Management
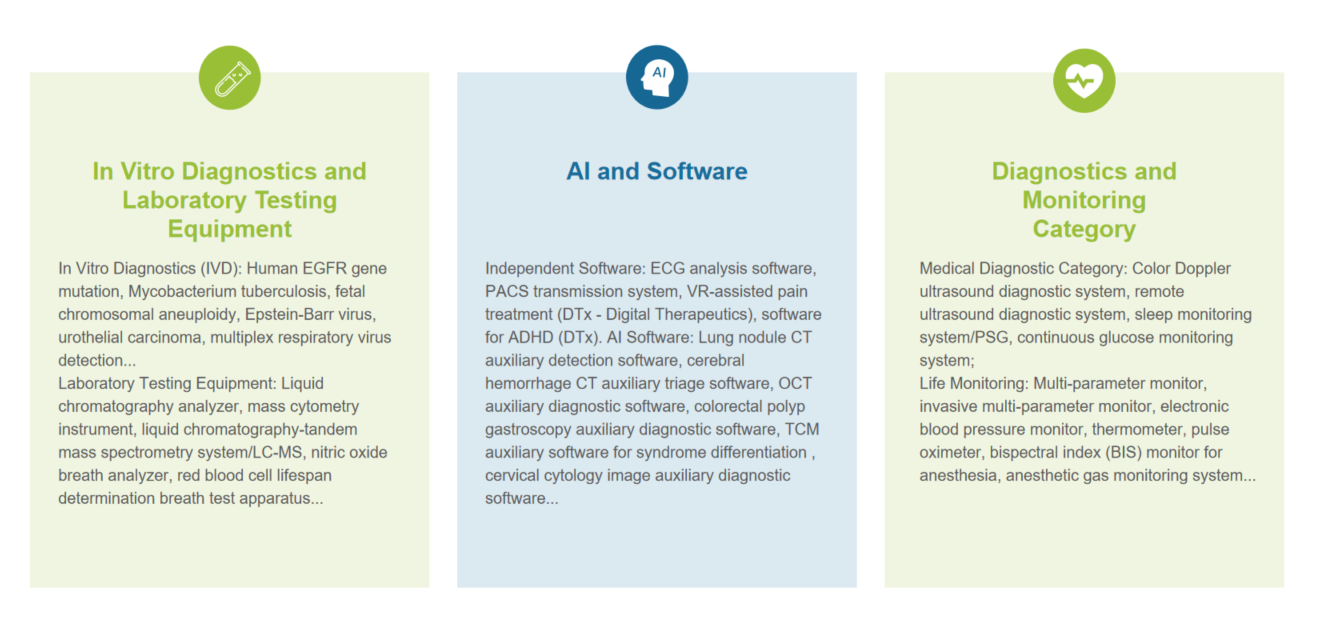
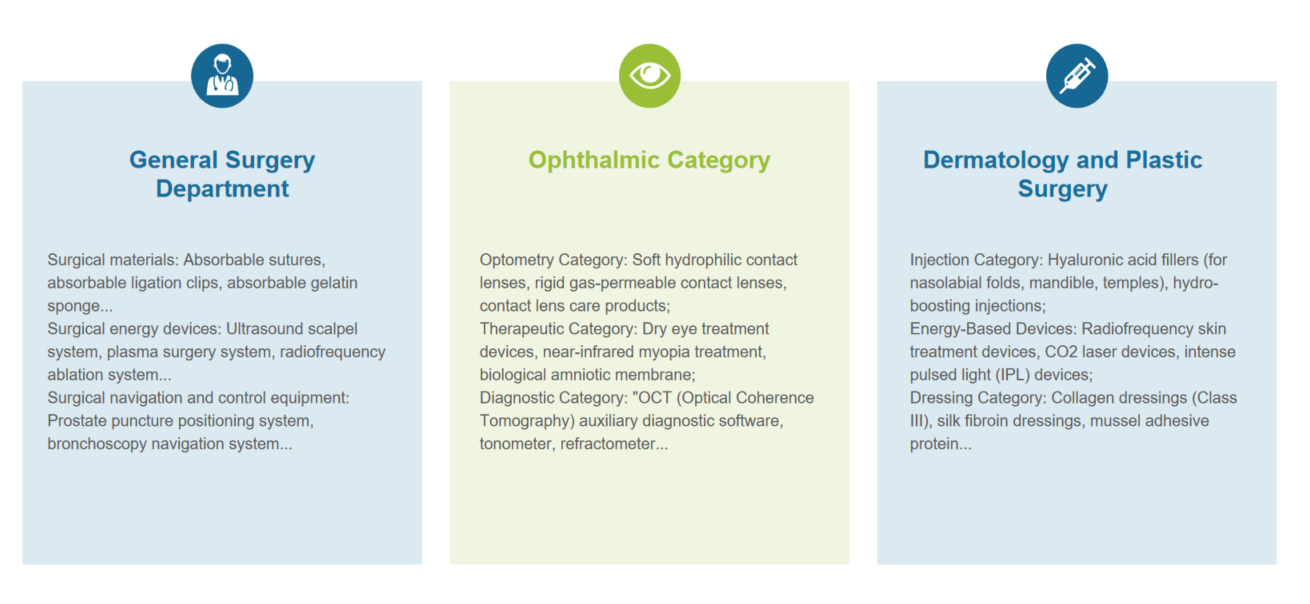
Case Sharing
Contact Us